Producing safe and wholesome food is one of our top priorities. Food safety and quality assurance are embedded in the codes of practice at all Sedef production sites. We are committed to the ISO 9001 standard for quality-oriented organisations, and all Sedef facilities are ISO 9001 certified .The only exceptions are those sites that have been part of Sedef for less than three years.
Our work on food safety covers diverse areas and processes:
All our sites have incorporated the Sedef-HACCP system, which is globally accepted. When it comes to slaughtering, there are two key principles for assuring food safety related to these zoonotic hazards. We prioritise these in our daily operations:
To verify compliance with food safety and customer requirements, all production sites are certified according to the rules of the Global Food Safety Initiative (GFSI). The two food safety private standards we use us are the International Featured Standards Food (IFS Food) and the British Retail Consortium (BRC) standard.
Our job is to create balanced food supply chains that are demand-driven and relevant for all partnersaFood safety and quality assurance are embedded in the codes of practice at all Vion production sites
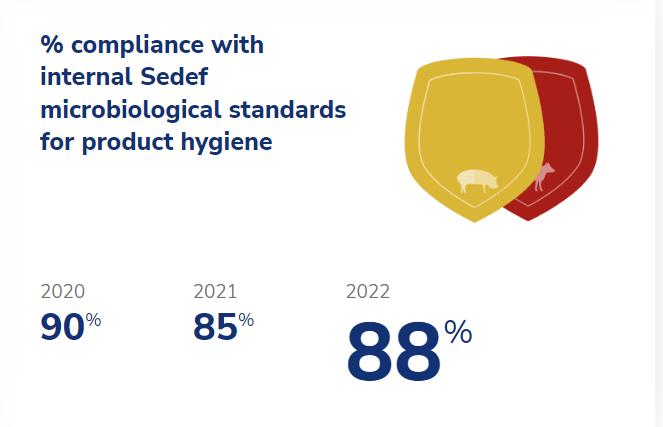
At Sedef, we recognize that products made from live animals inevitably contain microorganisms, as this is a natural part of the environment. However, we monitor the number of microorganisms as a key performance indicator to ensure that our work practices are as hygienic as possible. Our internal microbiological standard is based on the number of bacteria from the Enterobacteriacea bacterial family found during the final stages of production, when products have been handled the most. Although there may be natural variation in bacterial counts and product origin, we are committed to continuously improving our way of working and minimizing any microbiological food safety risks. In 2023, our objective is to have 90% of our pork and beef products comply with our internal standard.
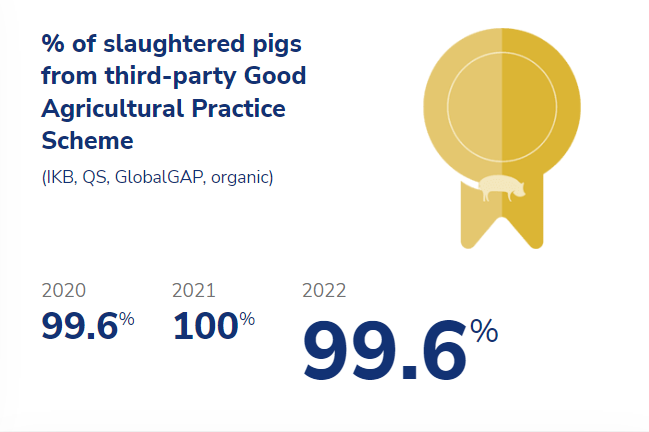
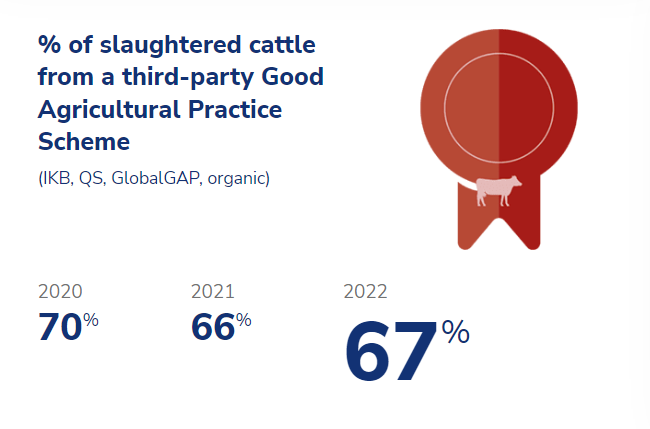
To mitigate risks in our supply chain, we require our suppliers to adhere to and certify their activities under a Good Agricultural Practice (GAP) scheme, such as GlobalGAP, the German QS (Qualität und Sicherheit), the Dutch Integrale Keten Beheersing (IKB) or Holland Varken. These schemes are independently organized and on-farm audits are carried out by independent certification bodies. As a major stakeholder, Sedef is committed to contributing to the design and organization of these schemes to ensure that our suppliers meet our high standards for quality and safety.
In 2022, no incidents were recorded in which Sedef products had a negative impact on consumers. In fact, several food safety interventions, such as continuous improvements in sanitary checks and hygienic working, which were built on the Sedef-HACCP programme, clearly showed an improvement when it comes to reducing human exposure to relevant hazards.
Sedef recognises Listeria monocytogenes as a relevant food safety hazard and we routinely monitor our products and the production environment for its presence. Listeria can occur anywhere in the environment and can multiply at refrigerator temperatures. Young children, pregnant women, the elderly and immunocompromised people are especially at risk of illness should they eat contaminated foods. In 2022, we began a quantitative microbial risk assessment (QMRA) specifically on Listeria in ready-to-eat food to ensure we have data-driven and science-based control in all our relevant production sites.
Ready-to-eat foods in which listeria can multiply are subjected to a so-called negative release programme: multiple samples of the product are taken from each batch and screened for the presence of listeria. The batch is released only when the bacterium is not detected in the samples.
We took several measures to control COVID-19 in our production premises
During 2022, we maintained our measures to control COVID-19 in our production premises. The benefits of vaccination and booster vaccinations were communicated to all employees and incentives were given to those that wanted to get vaccinated to do so. In addition to labour-related measures, we carried out product controls. This involved taking multiple samples of packaged products and outer packaging materials to check that COVID-19 was not present. The results of this extensive sampling showed that Sedef products did not contribute to the spread of COVID-19. Furthermore, no spreading events, as observed in 2021, were observed in Sedef locations in 2022.
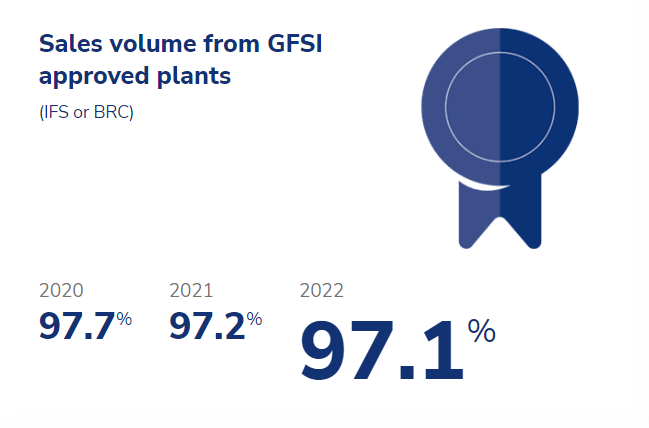
The percentage of sales from GFSI-approved plants was slightly lower in 2022 and 2021 compared to 2020 because we still have one production site that is not GFSI approved. As that site’s sales volume share increased, the GFSI percentage declined slightly.
The norms applied in Sedef’s internal microbiological standards for product hygiene are substantially stricter than those required by legislation. This ensures that legal compliance is guaranteed at all times. Given the natural variation in bacterial counts, it is unlikely that all samplings will always comply to our strict internal standards. However, by striving for a percentage that is as high as possible, we can achieve a continuous improvement in food safety.
© 2010 – 2023 Sedef SA. All right reserved.